Pogil properties of covalent bonds – In the realm of chemistry, the study of covalent bonds unravels the intricacies of molecular interactions. Covalent bonds, with their unique properties and fundamental role in shaping molecular geometry and reactivity, form the cornerstone of this exploration.
This comprehensive guide delves into the fascinating world of covalent bonds, examining their formation, stability, and impact on molecular structure and properties. From the sharing of electrons to the applications of covalent bonding in diverse fields, this discourse provides a captivating journey into the heart of chemical bonding.
Properties of Covalent Bonds: Pogil Properties Of Covalent Bonds
Covalent bonds are a unique type of chemical bond that involves the sharing of electron pairs between atoms. This type of bond is distinct from ionic bonds, where one atom transfers electrons to another, and metallic bonds, where electrons are delocalized across a metal lattice.Covalent
bonds are characterized by their strength and stability, which arise from the mutual attraction between the shared electrons and the positively charged nuclei of the bonded atoms. The strength of a covalent bond is influenced by several factors, including the number of shared electron pairs, the electronegativity of the bonded atoms, and the size of the atoms involved.
Factors Influencing Covalent Bond Strength
The strength of a covalent bond is primarily determined by the number of shared electron pairs. A single bond involves the sharing of one electron pair, a double bond involves the sharing of two electron pairs, and a triple bond involves the sharing of three electron pairs.
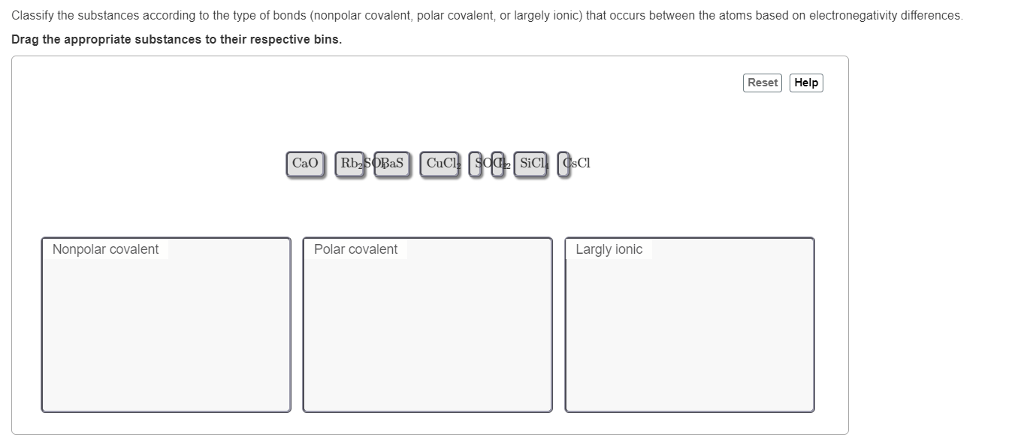
The greater the number of shared electron pairs, the stronger the covalent bond.Electronegativity, a measure of an atom’s ability to attract electrons, also plays a role in covalent bond strength. When two atoms with similar electronegativities bond, the electrons are shared equally between them, resulting in a nonpolar covalent bond.
However, when two atoms with different electronegativities bond, the electrons are drawn more towards the more electronegative atom, resulting in a polar covalent bond. Polar covalent bonds are typically weaker than nonpolar covalent bonds.Finally, the size of the bonded atoms can influence covalent bond strength.
Larger atoms have a greater distance between their nuclei, which reduces the overlap between their electron clouds. This reduced overlap leads to a weaker covalent bond.
Formation and Breaking of Covalent Bonds
Covalent bonds form the foundation of molecular structures, holding atoms together through the sharing of electrons. Understanding their formation and breaking is crucial for comprehending chemical reactions and molecular behavior.
Formation of Covalent Bonds
Covalent bond formation occurs when two atoms approach each other and their valence electrons interact. Each atom contributes one or more electrons to form a shared pair, creating a region of electron density between the nuclei. This electron pair is attracted to both nuclei, resulting in a strong electrostatic force that holds the atoms together.
Breaking of Covalent Bonds
Covalent bonds can be broken when sufficient energy is applied to overcome the attractive forces holding the atoms together. This energy can come from various sources, such as heat, light, or chemical reactions. When a covalent bond breaks, the shared electron pair is separated, and the atoms become independent entities.
The strength of a covalent bond is determined by several factors, including the electronegativity of the atoms involved, the number of shared electrons, and the bond length. These factors influence the amount of energy required to break the bond.
Molecular Geometry and Hybridization
Covalent bonding influences molecular geometry, which is the spatial arrangement of atoms within a molecule. To predict molecular geometry, we use the Valence Shell Electron Pair Repulsion (VSEPR) theory, which states that electron pairs in a molecule will adopt an arrangement that minimizes repulsion between them.
Hybridization
Hybridization is a theoretical concept that combines atomic orbitals to form new hybrid orbitals with different shapes and energies. Hybridization occurs when the central atom in a molecule has multiple electron pairs to bond with. The hybridization of atomic orbitals determines the molecular geometry and the bond angles between atoms.
Common types of hybridization include:
- sp hybridization: Two atomic orbitals (one s and one p) combine to form two sp hybrid orbitals that are linear (180° bond angle).
- sp 2hybridization: Three atomic orbitals (one s and two p) combine to form three sp 2hybrid orbitals that are trigonal planar (120° bond angles).
- sp 3hybridization: Four atomic orbitals (one s and three p) combine to form four sp 3hybrid orbitals that are tetrahedral (109.5° bond angles).
Polarity and Electronegativity
In covalent bonds, polarity refers to the uneven distribution of electrons between atoms. Electronegativity is the measure of an atom’s ability to attract electrons towards itself in a chemical bond.
Electronegativity differences between atoms in a covalent bond create a polar covalent bond, where one atom has a partial positive charge and the other has a partial negative charge. The greater the electronegativity difference, the more polar the bond will be.
Polarity and Properties of Covalent Compounds
The polarity of covalent bonds influences the properties of the compounds they form:
- Solubility:Polar covalent compounds tend to be soluble in polar solvents, such as water, while nonpolar covalent compounds are soluble in nonpolar solvents, such as oil.
- Boiling Point:Polar covalent compounds generally have higher boiling points than nonpolar covalent compounds due to stronger intermolecular forces.
- Melting Point:Polar covalent compounds generally have higher melting points than nonpolar covalent compounds due to stronger intermolecular forces.
- Reactivity:Polar covalent compounds are more reactive than nonpolar covalent compounds because the partial charges make them more susceptible to attack by other molecules.
Applications of Covalent Bonding
Covalent bonding is a fundamental force that plays a crucial role in various scientific disciplines and technological advancements. The formation of covalent bonds between atoms results in the creation of molecules with distinct properties and functionalities. These molecules are the building blocks of many materials and biological systems.
Materials Science, Pogil properties of covalent bonds
Covalent bonding is essential in the development of advanced materials with tailored properties. For instance, in the field of nanotechnology, covalent bonding is utilized to create carbon nanotubes and graphene, which possess exceptional strength, electrical conductivity, and thermal stability. These materials find applications in electronics, optics, and energy storage.
Biotechnology
Covalent bonding is the foundation of biological molecules, such as proteins, carbohydrates, and nucleic acids. These molecules are responsible for the structure, function, and regulation of living organisms. In biotechnology, covalent bonding is harnessed to design and synthesize new drugs, enzymes, and other therapeutic agents.
Organic Chemistry
Covalent bonding is central to organic chemistry, the study of carbon-based compounds. Organic molecules exhibit a vast array of properties and reactivities due to the diverse ways in which carbon atoms can form covalent bonds with other atoms, including hydrogen, oxygen, nitrogen, and halogens.
Organic molecules are essential components of pharmaceuticals, plastics, fuels, and many other products.
Question & Answer Hub
What are the key factors that determine the strength of a covalent bond?
The strength of a covalent bond is primarily influenced by the electronegativity difference between the bonded atoms, the bond length, and the number of shared electrons.
How does hybridization contribute to the shape of molecules?
Hybridization involves the mixing of atomic orbitals to create new hybrid orbitals with specific shapes. These hybrid orbitals determine the geometry and bonding angles of molecules.
What is the relationship between polarity and electronegativity?
Polarity arises when there is an uneven distribution of electrons in a covalent bond due to differences in electronegativity. Electronegativity measures the ability of an atom to attract electrons.