Embark on a journey to conquer molarity calculations with the Molarity Practice Worksheet Answer Key. This comprehensive guide unveils the intricacies of molarity, empowering you to navigate the world of chemistry with confidence.
Within these pages, you’ll find a treasure trove of practice problems, step-by-step solutions, and real-world applications that will transform you into a molarity maestro.
Molarity Definition and Formula: Molarity Practice Worksheet Answer Key
Molarity is a measure of the concentration of a solution, specifically the number of moles of solute per liter of solution. It is a crucial concept in chemistry, as it allows us to determine the amount of a substance present in a given volume of solution.
The formula for calculating molarity (M) is:
M = moles of solute / liters of solution
Where:
- Moles of solute: The number of moles of the solute present in the solution.
- Liters of solution: The volume of the solution in liters.
To calculate molarity, simply divide the number of moles of solute by the volume of the solution in liters.
Molarity Practice Problems, Molarity practice worksheet answer key
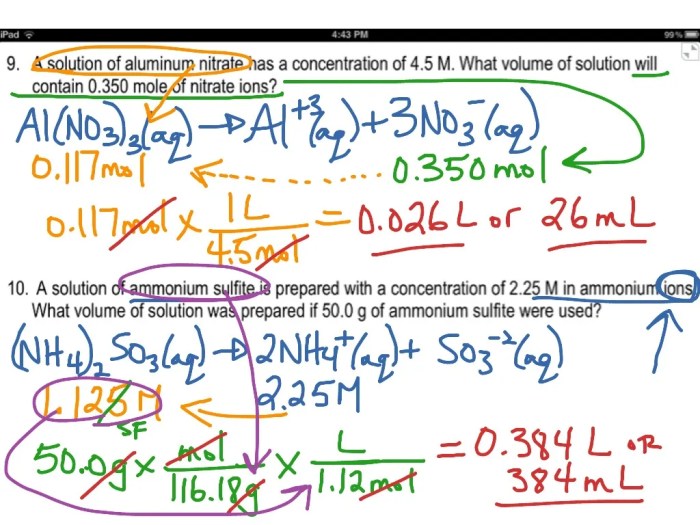
Problem 1:Calculate the molarity of a solution containing 0.5 moles of NaCl in 2 liters of solution.
Solution:
- M = 0.5 moles / 2 liters = 0.25 M
Problem 2:How many moles of NaOH are present in 500 mL of a 0.1 M solution?
Solution:
- Convert 500 mL to liters: 500 mL = 0.5 liters
- Moles of NaOH = Molarity x Volume (in liters)
- Moles of NaOH = 0.1 M x 0.5 liters = 0.05 moles
Dilution Calculations
Dilution is the process of adding more solvent to a solution, which decreases the concentration of the solute. The formula for calculating the molarity of a diluted solution is:
M1V1 = M2V2
Where:
- M1: Initial molarity of the solution.
- V1: Initial volume of the solution.
- M2: Final molarity of the diluted solution.
- V2: Final volume of the diluted solution.
To perform a dilution calculation, simply rearrange the formula to solve for the desired variable.
Real-World Applications of Molarity
Molarity is a versatile concept with numerous applications in various fields, including:
- Medicine:Molarity is used to determine the concentration of drugs in solutions, ensuring accurate dosage and treatment.
- Environmental Science:Molarity helps monitor the concentration of pollutants in water and soil, aiding in environmental protection.
- Industrial Chemistry:Molarity is crucial in controlling the concentration of reactants and products in chemical processes, optimizing production and minimizing waste.
Question Bank
What is the formula for calculating molarity?
Molarity = Moles of Solute / Liters of Solution
How do I solve dilution problems?
M1V1 = M2V2 (Initial concentration x Initial volume = Final concentration x Final volume)
What are the real-world applications of molarity?
Molarity is used in medicine to determine drug dosages, in environmental science to analyze water quality, and in industrial chemistry to control chemical reactions.