Acids and bases worksheet answers provide comprehensive explanations and solutions to essential concepts in chemistry. These answers delve into the fundamental definitions, properties, and applications of acids and bases, empowering students to master this crucial topic.
The following guide offers a thorough overview of acids and bases, covering topics such as pH and pOH calculations, acid-base titrations, and acid-base equilibria. By exploring these concepts, students gain a deeper understanding of the chemical interactions that shape our world.
Acids and Bases Definitions: Acids And Bases Worksheet Answers

Acids and bases are two fundamental chemical concepts that play a crucial role in various scientific disciplines and everyday life. The Bronsted-Lowry theory of acids and bases defines acids as proton donors and bases as proton acceptors.
Acids release H+ ions (protons) in aqueous solutions, while bases accept H+ ions. Examples of common acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH). Examples of common bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
Acids typically have a sour taste and can react with metals to produce hydrogen gas. Bases are usually bitter and slippery to the touch and can react with acids to produce salts and water.
pH and pOH Calculations
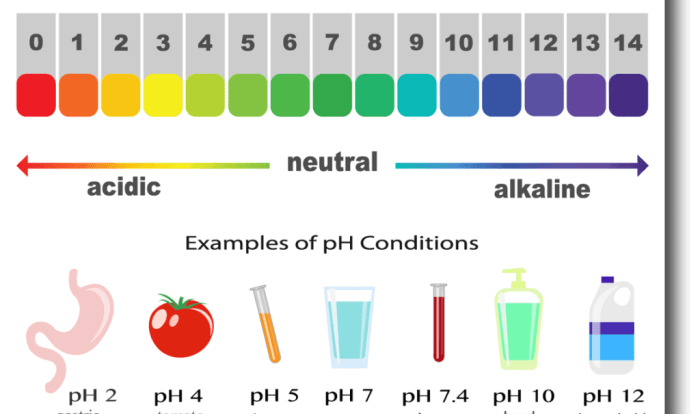
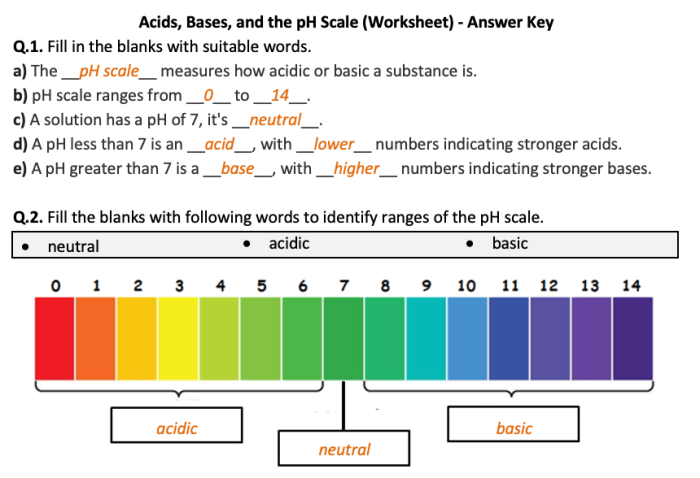
pH and pOH are logarithmic scales used to measure the acidity or basicity of a solution. pH is defined as the negative logarithm of the hydrogen ion concentration ([H+]), while pOH is the negative logarithm of the hydroxide ion concentration ([OH-]).
The relationship between pH and pOH is: pH + pOH = 14.
To calculate the pH of a solution, use the following formula: pH = -log[H+]. To calculate the pOH, use the formula: pOH = -log[OH-].
For example, a solution with an [H+] of 1 x 10^-7 M has a pH of 7. A solution with an [OH-] of 1 x 10^-4 M has a pOH of 4.
Acid-Base Titrations
An acid-base titration is a laboratory technique used to determine the concentration of an unknown acid or base. The procedure involves adding a known volume of a standardized acid or base to a known volume of the unknown solution until the reaction is complete.
The equivalence point of a titration is reached when the moles of acid added are equal to the moles of base present. The equivalence point can be determined using an indicator, which changes color at a specific pH.
The concentration of the unknown solution can be calculated using the following formula: M1V1 = M2V2, where M1 and V1 are the concentration and volume of the known solution, and M2 and V2 are the concentration and volume of the unknown solution.
Acid-Base Equilibria
Acid-base equilibria are chemical reactions in which an acid and a base react to form a conjugate acid-base pair. The equilibrium constant for an acid-base reaction is known as the dissociation constant, Ka.
The common ion effect is a phenomenon that occurs when a common ion is added to a solution of a weak acid or base, causing a decrease in the dissociation of the acid or base.
The buffer effect is a phenomenon that occurs when a weak acid and its conjugate base are present in a solution, resulting in a resistance to changes in pH.
Applications of Acids and Bases
Acids and bases have numerous applications in everyday life and various industries.
In everyday life, acids are used in batteries, fertilizers, and cleaning products. Bases are used in soaps, detergents, and antacids.
In industries, acids are used in the production of chemicals, textiles, and food. Bases are used in the production of paper, glass, and pharmaceuticals.
Acids and bases also play a crucial role in environmental processes, such as the regulation of pH in soil and water.
FAQ Resource
What is the Bronsted-Lowry theory of acids and bases?
The Bronsted-Lowry theory defines acids as substances that donate protons (H+) and bases as substances that accept protons.
How do you calculate pH from H+ concentration?
pH = -log[H+].
What is the purpose of an acid-base titration?
Acid-base titrations are used to determine the concentration of an unknown acid or base by neutralizing it with a solution of known concentration.