Mixtures compounds and elements worksheet – The Mixtures, Compounds, and Elements Worksheet provides an in-depth exploration of the fundamental concepts of chemistry, offering a comprehensive understanding of the nature and properties of these essential substances. This worksheet delves into the definitions, types, properties, and methods of separating mixtures, as well as the formation and characteristics of compounds and elements.
Through a systematic approach, this worksheet guides learners through the intricacies of chemical substances, equipping them with a solid foundation in the field of chemistry.
Mixtures, Compounds, and Elements
Matter is anything that takes up space and can be weighed. Matter can be classified into three categories: mixtures, compounds, and elements.
A mixture is a combination of two or more chemical substances that are not chemically bonded. The substances retain their identity and are mixed in different forms, for example, solutions, suspensions, or colloids.
A compound is a substance composed of two or more elements chemically bonded together in fixed proportions. Compounds have different properties from their constituent elements.
An element is a substance that cannot be broken down into simpler substances by chemical means. Elements are the basic building blocks of matter and are represented by the symbols in the periodic table.
Types of Mixtures, Mixtures compounds and elements worksheet
Mixtures can be classified into two main types:
- Homogeneous mixturesare mixtures in which the components are evenly distributed throughout the mixture. Solutions are homogeneous mixtures.
- Heterogeneous mixturesare mixtures in which the components are not evenly distributed throughout the mixture. Suspensions and colloids are heterogeneous mixtures.
Properties of Mixtures
The properties of a mixture are determined by the properties of its components.
- Physical propertiesof mixtures, such as color, density, and boiling point, are typically an average of the properties of the components.
- Chemical propertiesof mixtures are determined by the chemical properties of the components. For example, a mixture of flammable and non-flammable substances will be flammable.
Methods for Separating Mixtures
There are a variety of methods that can be used to separate mixtures, including:
- Filtrationis used to separate solids from liquids.
- Distillationis used to separate liquids with different boiling points.
- Chromatographyis used to separate substances based on their different rates of movement through a stationary phase.
Formation of Compounds
Compounds are formed when atoms of different elements are chemically bonded together.
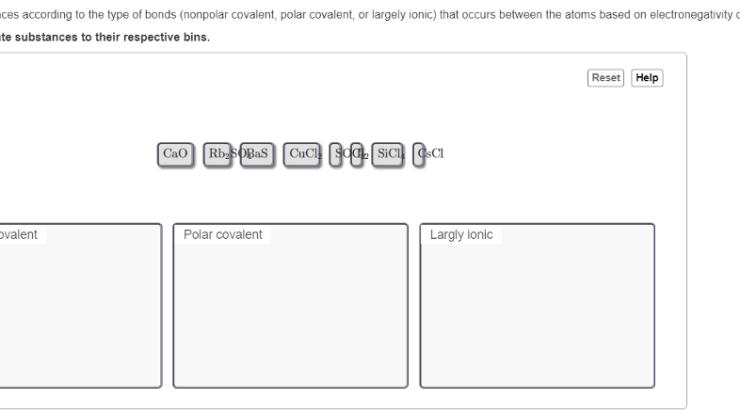
- Ionic bondingoccurs when one atom transfers electrons to another atom, creating oppositely charged ions that are attracted to each other.
- Covalent bondingoccurs when atoms share electrons, creating a covalent bond between them.
- Metallic bondingoccurs when metal atoms share their valence electrons in a sea of electrons.
Properties of Compounds
The properties of a compound are different from the properties of its constituent elements.
- Physical propertiesof compounds, such as color, density, and melting point, are typically different from the properties of the elements that make them up.
- Chemical propertiesof compounds are determined by the chemical properties of the elements that make them up.
Types of Elements
Elements can be classified into three main types:
- Metalsare shiny, malleable, and ductile.
- Nonmetalsare dull, brittle, and poor conductors of electricity.
- Metalloidshave properties of both metals and nonmetals.
Properties of Elements
The properties of an element are determined by the number of protons in its nucleus.
- Physical propertiesof elements, such as color, density, and melting point, are typically different from the properties of compounds and mixtures.
- Chemical propertiesof elements are determined by the number of electrons in the element’s outermost energy level.
Quick FAQs: Mixtures Compounds And Elements Worksheet
What is the difference between a mixture and a compound?
A mixture is a combination of two or more chemical substances that retain their individual identities and can be easily separated by physical means. A compound, on the other hand, is a substance formed by the chemical combination of two or more elements in fixed proportions, resulting in a new substance with unique properties.
What are the different types of mixtures?
Mixtures can be classified into two main types: homogeneous and heterogeneous. Homogeneous mixtures are uniform throughout, with the components evenly distributed. Heterogeneous mixtures, on the other hand, are non-uniform and exhibit visible differences in composition.
How can mixtures be separated?
There are various methods for separating mixtures, including filtration, distillation, chromatography, and magnetic separation. The choice of method depends on the properties of the mixture and the desired separation.